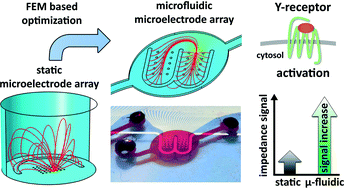
Lab-on-a-chip devices that combine, e.g. chemical synthesis with integrated on-chip analytics and multi-compartment organ-on-a-chip approaches, are a fast and attractive evolving research area. While integration of appropriate cell models in microfluidic setups for monitoring the biological activity of synthesis products or test compounds is already in focus, the integration of label-free bioelectronic analysis techniques is still poorly realized. In this context, we investigated the capabilities of impedance spectroscopy as a non-destructive real-time monitoring technique for adherent cell models in a microfluidic setup. While an initial adaptation of a microelectrode array (MEA) layout from a static setup revealed clear restrictions in the application of impedance spectroscopy in a microfluidic chip, we could demonstrate the advantage of a FEM simulation based rational MEA layout optimization for an optimum electrical field distribution within microfluidic structures. Furthermore, FEM simulation based analysis of shear stress and time-dependent test compound distribution led to identification of an optimal flow rate. Based on the simulation derived optimized microfluidic MEA, comparable impedance spectra characteristics were achieved for HEK293A cells cultured under microfluidic and static conditions. Furthermore, HEK293A cells expressing Y1 receptors were used to successfully demonstrate the capabilities of impedimetric monitoring of cellular alterations in the microfluidic setup. More strikingly, the maximum impedimetric signal for the receptor activation was significantly increased by a factor of 2.8. Detailed investigations of cell morphology and motility led to the conclusion that cultivation under microfluidic conditions could lead to an extended and stabilized cell–electrode interface.
A novel microfluidic microelectrode chip for a significantly enhanced monitoring of NPY-receptor activation in live mode