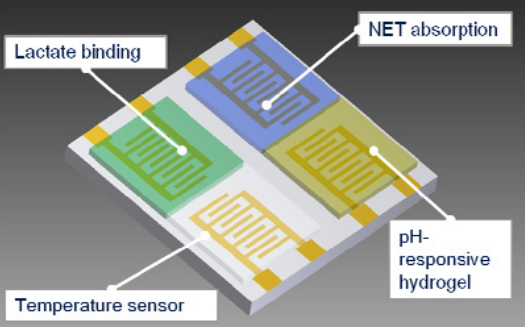
Wound infection monitoring is a challenging task. It is only solvable by designing an integrable and cost-efficient sensor which measures a relevant set of parameters. One viable parameter is the formation of neutrophil extracellular traps (NETs). Their task is trapping pathogens in the wound. A wound infection results in massive release of them which can be detected with impedimetric methods. Our investigations focused on the characterization of the biological process with an in vitro model. The model environment is a cell culture with neutrophil granulocytes cultured on interdigitated electrodes which represent the sensor surface. Detected impedance changes caused by NET-formation were in the range of 35% and even higher. This implies that impedance measurements are suitable for NET detection. We derived a measurement and evaluated it by differing conditions like changing stimulation agent and varying the cell number. For both conditions the results of impedance and phase angle deviation can be confirmed. In combination with other parameters a sensor can be designed for specific detection of wound infections. These aspects are integrated in our sensor concept.
Infection Monitoring in Wounds